UAN Solutions
Urea-ammonium nitrate (UAN) solutions are mixtures of urea, ammonium nitrate, and water in various proportions. All common UAN solutions (28%, 30% and 32%) are formulated to contain 50% of actual N as amide, (from urea), 25% as ammonium (from ammonium nitrate), and 25% as nitrate (from ammonium nitrate).
Soil Reactions – The urea portion of UAN solutions reacts just as dry urea does (see previous section on urea). If applied on the surface, the amide-N in the solution may incur losses due to volatilization when urease hydrolysis releases NH3. But if UAN is incorporated by tillage or sufficient water, the NH3, quickly reacts with soil water to form NH4+. This ammonium, as well as the ammonium N derived from ammonium nitrate in the solution, adheres to soil components at the application site and is not subject to loss in the short term. Like N applied as anhydrous ammonia, this N will eventually be taken up by plants in the ammonium form, or if not, eventually converted to nitrate by soil bacteria.
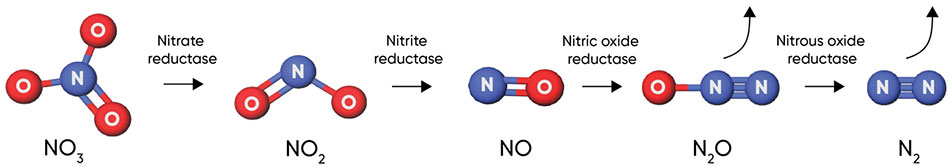
The remaining 25% of nitrogen in UAN solutions is in the nitrate (NO3-) form. Because it is negatively charged, it will not adhere to clay and organic matter particles (which are also negatively charged) but rather, will exist as an anion in the soil solution. Because it moves with water, it is easily taken up by plant roots, but is also subject to losses by leaching and denitrification.